Control System Optimization and Testing
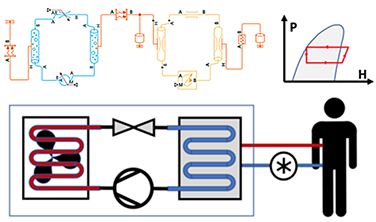
Supporting the push for FDA regulatory submission, provided services for feedback control system optimization and testing for a medical device managing patient body temperature. The effort included developing physics based-models of the medical device and patient response to treatment from empirical data, and extensive testing.
The Model Based Design process used Matlab/Simulink for simulating the feedback control system, and response of the patient to treatment. A Simscape physics-based model was also developed and matched to experimental data to simulate the response of the medical device to control system commands, including two-phase refrigeration, heat flow, and thermal fluid dynamics.
System testing was also a significant phase of the effort. Results included not only test results but also stronger testing protocols and logging of the majority of defects found during the push to submission.
The project followed the 60601 safety standard.
Mechanical Engineering Workflow Automation
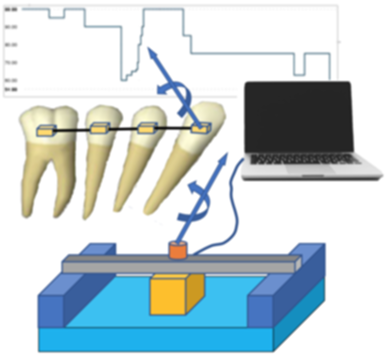
A desktop application for automating dental mechanical engineering experiments was developed following the full life cycle software development process, including requirements gathering, multiple phases of prototyping, and delivery of the compiled application to users in the lab. An 80% reduction in engineering time was achieved. Quality and consistency of experimental data was also improved by the standardized process.
The application aquired live data from and array force and moment sensors in the test fixture, which enabled 2D and 3D visualization of an experiment in progress. Unexpected results were immediately visible, allowing them to addressed as they occurred, improving quality avoiding repeated experiments.
Power Electronics Prototyping
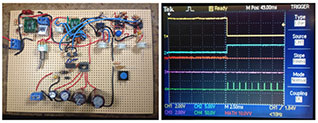
Proof of concept prototyping of power electronics including LiIon battery management, a boost converter, and bipolar and field effect transistors, to operate a solenoid using a button battery as the energy source for a consumer product.
Aerial Image Analysis for Agriculture
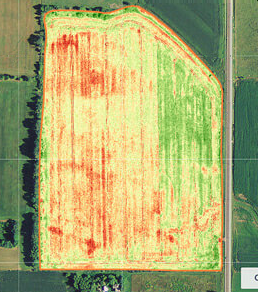
Aerial imagery from drones combined with state of the art image processing technology was used to provide farms with quantitative data never before available for predicting yield of fields on the farm, analysing the health of the crop, and provide input for planning for future seasons. The project made heavy use of 3D machine vision technology in translating the large batch of imagery into reports for the farm.
Real-Estate Geospatial Database
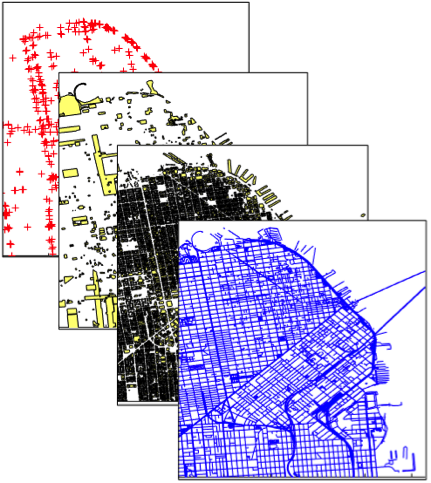
In support of startup in quiet mode seeking to become the counterpart of Zillow for finding real estate agents, an extensive database was created from both public and paid sources to support search and provide detailed information including summary statistics for agents, cities, zip codes, neighborhoods, school districts, and more. Data sources included real estate listings, sale transation, school test scores, census data, business statistics, Open Street Maps data, Zillow neighborhoods, and more. The effort included identifying data sources, data cleaning, integration of data from multiple sources, and generating summary statistics. All this taken together helps find agents that are have experince in local markets and with homes that are well matched to a perspective home buyer or seller.
Genomics Algorithm Validation
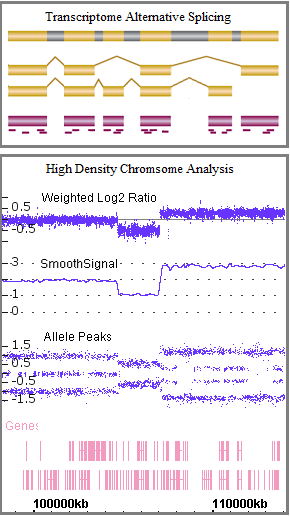
Validated genomics algorithms for microarray data analysis in support of numerous product releases. Validated algorithm output included expression log2 ratio, genotype calling, genome coverage reporting, copy number, loss of heterozygosity, mosaicism, and QC metrics. Delverables were test results for each release, and a repeatable process to provide surveillance of code in the version control system and quickly extend coverage to new features.
Microarrays for which validated algorithms processed data included Applied Biosystems™ Axiom™ catalog and myDesign™ custom arrays for human, animal, and plant genomes, GeneChip™ 500K for genotyping, CytoScan™ and OncoScan™ for cytogenetics, GeneChip™ U133 and miRNA for gene expression, and GeneChip™ WT for transcriptome and alternative splicing analysis. Customer software using validated algorithms included Array Power Tools, Transcriptome Analysis Console™ (TAC), and SNPolisher.
Gene Expression Data Browser
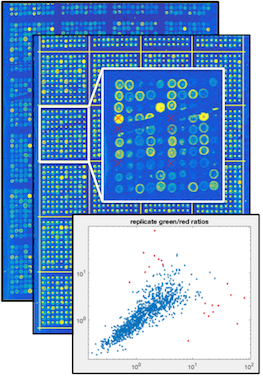
During the course of AB1700 whole human microarray development, a vast collection of experimental data was generated as the platform moved from prototype to the marketplace. It became a challenge for the team to keep the data organized, and insure visibility input data and results across the team for the experiments.
To solve this problem, a system was created to allow users to browse the data at the experiment level, then drill down into the data to images of the microarrays, then deeper the gridding and gene experession data that was generatated from the experiment. The data could be sliced and diced in multiple dimensions, some interesting to manufacturing, some interesting to the chemistry team, and some interesting to algorithm developers. The internally developed system served as a genome data warehouse, and a visualization system much like GeneSpring and Tableau before those products were widely available.
Batch Process Control Automation
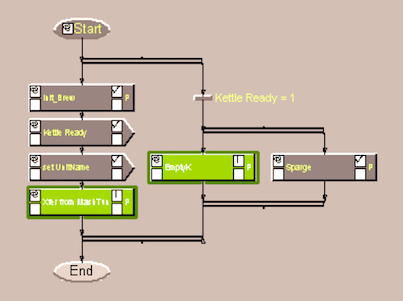
Batch Data Manager (BDM) is used to manage, display, and edit recipie diagrams used for batch process automation, as well as to monitor status, modify parameters, issue commands, and log events during batch execution from a Windows NT workstation. This product is now marketed as Symbatch NT by ABB.
During the development effort BDM was upgraded from version 4 to version 5, adding capability to modify parameters during batch execution, improved security, and numerous user interface feature improvements.
Regulatory Compliance Internet Database
As companies expand to workdwide markets, paper based systems that were manageable at one office did not scale well once the company expanded to multiple offices at international locations. To solve this problem for the regulatory group of a medical device manufacturer, an all electronic system accessible throughout the corporate network was developed and launched in support of 5 international offices. This enabled the company to streamline sharing of information and automate workflow worldwide.
Development from inception to delivery began by identifying hundreds of product requirements through interviews with stakeholders worldwide during a two phase prototyping process. The completed system was then validated through functional and system tests traceable back to requirements selected for implementation. Numerous living documents were maintained, including the project plan, schedule, requirements, design, validation plan and test results in accordance with FDA Good Manufacturing Practices (GMP) regulation 21CFR820.
Axiom, GeneChip, CytoScan, OncoScan, and myDesign are trademarks or registered trademarks of Affymetrix, Inc. Other product or brand names are trademarks or registered trademarks of their respective holders.
For Affymetrix product information please see:
Web: www.affymetrix.com